Significant tricuspid regurgitation is associated with adverse outcomes in patients with transthyretin amyloid cardiomyopathy
DOI:
https://doi.org/10.47487/apcyccv.v5i2.388Keywords:
Amyloidosis, Heart Failure, Tricuspid RegurgitationAbstract
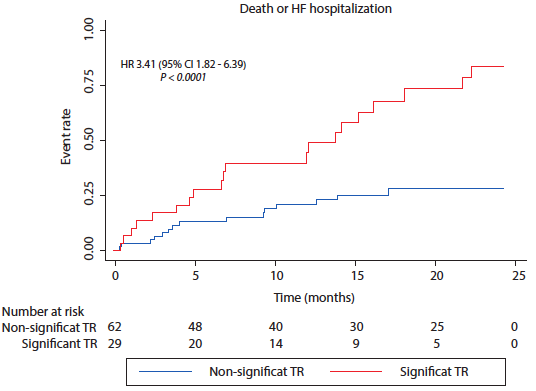
Objectives. Patients diagnosed with transthyretin amyloid cardiomyopathy (ATTR-CM) often experience poor outcomes due to the development of heart failure (HF). Tricuspid regurgitation (TR) has been found to be correlated with adverse outcomes in patients with HF. This study aims to assess whether the presence of significant TR is associated to adverse cardiac outcomes in patients diagnosed with ATTR-CM. Materials and methods. Retrospective study of ATTR-CM patients enrolled in the Institutional Registry of Amyloidosis (NCT01347047). Patients were categorized based on the presence of significant TR (moderate or severe according to current guidelines criteria) or absence of significant TR. All patients were followed up for 2 years to assess the incidence of the composite outcome of death or HF hospitalization. Results. A total of 93 ATTR-CM patients were included. The mean age at diagnosis was 82.5 [IQR 75 - 86] years, 86% were male, and the mean left ventricular ejection fraction was 52% [IQR 43 - 60]. Among them, 32.3% (n = 30) patients had significant TR. Patients with significant TR had higher NTpro-BNP values (5308 vs 2454, pg/mL, p = 0.004), and a lower left ventricular ejection fraction (44 vs. 56%, p = 0.0002) compared to patients without significant TR. The incidence of the primary outcome was higher in patients with significant TR (77% vs. 30%, p<0.001). In a multivariate Cox regression analysis, only NTpro-BNP, as a numerical variable (HR 1.00, 95% CI 1.00005-1.0002, p = 0.001), and significant TR (HR 2.23, 95% CI 1.12-4.42, p=0.021) were independently associated with the composite outcome of death or HF hospitalization. Conclusions. In patients diagnosed with ATTR-CM, the presence of significant TR was associated with worse outcomes.
Downloads
References
Martinez-Naharro A, Hawkins PN, Fontana M. Cardiac amyloidosis. Clin Med (Lond). 2018;1;18(Suppl 2):s30-s35. doi: 10.7861/clinmedicine.18-2-s30.
Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ Heart Fail. 2019;12(9):e006075. doi: 10.1161/CIRCHEARTFAILURE.119.006075.
Patel KS, Hawkins PN. Cardiac amyloidosis: where are we today? J Intern Med. 2015;278(2):126-44. doi: 10.1111/joim.12383..
Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation. 2017;135(14):1357-1377. doi: 10.1161/CIRCULATIONAHA.116.024438.
Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;11;73(22):2872-2891. doi: 10.1016/j.jacc.2019.04.003.
Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J Am Coll Cardiol. 2016;68(10):1014-20. doi: 10.1016/j.jacc.2016.06.033.
Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;4;126(10):1286-300. doi: 10.1161/CIRCULATIONAHA.111.078915.
Aluru JS, Barsouk A, Saginala K, Rawla P, Barsouk A. Valvular Heart Disease Epidemiology. Med Sci (Basel). 2022;10(2):32. doi: 10.3390/medsci10020032.
Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, et al. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc Imaging. 2019;12(3):433-442. doi: 10.1016/j.jcmg.2018.06.014.
Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43(3):405-9. doi: 10.1016/j.jacc.2003.09.036.
Benfari G, Antoine C, Miller WL, Thapa P, Topilsky Y, Rossi A, et al. Excess Mortality Associated With Functional Tricuspid Regurgitation Complicating Heart Failure With Reduced Ejection Fraction. Circulation. 2019;140(3):196-206. doi: 10.1161/CIRCULATIONAHA.118.038946.
Harada T, Obokata M, Omote K, Iwano H, Ikoma T, Okada K, et al. Functional Tricuspid Regurgitation and Right Atrial Remodeling in Heart Failure With Preserved Ejection Fraction. Am J Cardiol. 2022;162:129-135. doi: 10.1016/j.amjcard.2021.09.021.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e35-e71. doi: 10.1161/CIR.0000000000000932.
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561-632. doi: 10.1093/eurheartj/ehab395.
Fagot J, Lavie-Badie Y, Blanchard V, Fournier P, Galinier M, Carrié D, et al. Impact of tricuspid regurgitation on survival in patients with cardiac amyloidosis. ESC Heart Fail. 2021;8(1):438-446. doi: 10.1002/ehf2.13093.
Prihadi EA, Delgado V, Leon MB, Enriquez-Sarano M, Topilsky Y, Bax JJ. Morphologic Types of Tricuspid Regurgitation: Characteristics and Prognostic Implications. JACC Cardiovasc Imaging. 2019;12(3):491-499. doi: 10.1016/j.jcmg.2018.09.027.
Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098. doi: 10.1161/JAHA.113.000098.
Lane T, Fontana M, Martinez-Naharro A, Quarta CC, Whelan CJ, Petrie A, et al. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation. 2019;140(1):16-26. doi: 10.1161/CIRCULATIONAHA.118.038169.
Cappelli F, Martone R, Gabriele M, Taborchi G, Morini S, Vignini E, et al. Biomarkers and Prediction of Prognosis in Transthyretin-Related Cardiac Amyloidosis: Direct Comparison of Two Staging Systems. Can J Cardiol. 2020;36(3):424-431. doi: 10.1016/j.cjca.2019.12.020.
Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. doi: 10.1056/NEJMoa1805689.
Tabata N, Sugiura A, Tsujita K, Nickenig G, Sinning JM. Percutaneous interventions for mitral and tricuspid heart valve diseases. Cardiovasc Interv Ther. 2020;35(1):62-71. doi: 10.1007/s12928-019-00610-z.
Braun D, Nabauer M, Orban M, Gross L, Englmaier A, Rösler D, et al. Transcatheter treatment of severe tricuspid regurgitation using the edge-to-edge repair technique. EuroIntervention. 2017;12(15):e1837-e1844. doi: 10.4244/EIJ-D-16-00949.
Schueler R, Hammerstingl C, Werner N, Nickenig G. Interventional direct annuloplasty for functional tricuspid regurgitation. JACC Cardiovasc Interv. 2017;10(4):415-416. doi: 10.1016/j.jcin.2016.10.033.
Nickenig G, Weber M, Schueler R, Hausleiter J, Näbauer M, von Bardeleben RS, et al. 6-month outcomes of tricuspid valve reconstruction for patients with severe tricuspid regurgitation. J Am Coll Cardiol. 2019;73(15):1905-1915. doi: 10.1016/j.jacc.2019.01.062.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 The journal is headline of the first publication, then the author giving credit to the first publication.

This work is licensed under a Creative Commons Attribution 4.0 International License.














