Congenital long QT syndrome
DOI:
https://doi.org/10.47487/apcyccv.v2i1.125Keywords:
Long QT syndrome, sudden death, pediatrics, Torsades de pointesAbstract
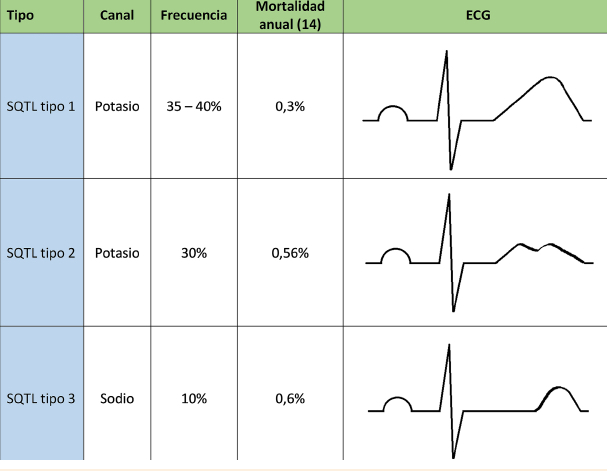
Congenital long QT syndrome (LQTS) represents a group of heart diseases of genetic origin characterized by prolongation of the QT interval and an abnormal T wave on the electrocardiogram (ECG). They can have a dominant or recessive expression, the latter associated with sensorineural deafness. In both cases, its clinical presentation is associated with recurrent syncope and sudden death as a consequence of ventricular tachycardia, specifically Torsades de Pointes. Currently they are classified according to the specific genetic defect, being able to compromise around 16 genes and almost 2000 mutations. It should be suspected in individuals with related symptoms, electrocardiographic findings, and family history. Management is based on the reduction or elimination of symptoms, and concomitantly the prevention of sudden death (SD), in those children with congenital deafness, the management requires the application of the otolaryngologist specialist's own measures. The cardiovascular management implies the modification of lifestyles, mainly the prohibition of competitive sports, including swimming, avoiding exposure to loud sounds or triggers. The medications used include beta-blockers, and more rarely flecainide, ranozaline, and verapamil; invasive management consists of the implantation of a cardioverter defibrillator or even left sympathetic denervation, each with its own risks and benefits. In any of the cases, we must avoid the circumstances that increase the QT interval, as well as carry out the appropriate analysis of the benefits and risks of each possible invasive measure.
Downloads
References
Brugada J, Blom N, Sarquella-Brugada G, Blomstrom-Lundqvist C, Deanfield J, Janousek J, et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. EP Eur. 2013;15(9):1337-82.
Isbister J, Semsarian C. Sudden cardiac death: an update. Intern Med J. 2019;49(7):826-33.
Jazayeri M-A, Emert MP. Sudden Cardiac Death. Med Clin North Am. 2019;103(5):913-30.
Sherwin ED, Berul CI. Sudden Cardiac Death in Children and Adolescents. Card Electrophysiol Clin. 2017;9(4):569-79.
Baltogiannis G, Conte G, Sieira J, De Ferrari GM, Brugada P. Editorial: Sudden Cardiac Death and Channelopathies. Front Cardiovasc Med. 2020;7:1-3.
Sarquella-Brugada G, Campuzano O, Brugada R. Trastornos del ritmo cardiaco más frecuentes en pediatría: síndrome de QT largo. Pediatr Integral 2012;16(8):617-621.
Wong AR. Clinical and genetic analysis of long QT syndrome in two Malay children. Med J Malasya 2019;74(4):341-343.
Haverkamp W. Kongenitales Long-QT-Syndrom. Herz Kardiovaskuläre Erkrank. 2007;32(3):201-205.
Kwok S, Liu AP, Chan CY, Lun K, Fung JL, et al. Clinical and genetic profile of congenital long QT syndrome in Hong Kong: a 20-year experience in paediatrics. Hong Kong Med J. 2018;24(6): 561-570.
Zullo A, Frisso G, Detta N, Sarubbi B, Romeo E, Cordella A, et al. Allelic Complexity in Long QT Syndrome: A Family-Case Study. Int J Mol Sci. 2017;18(8):1633.
Lahrouchi N, Tadros R, Crotti L, Mizusawa Y, Postema PG, Beekman L, et al. Transethnic Genome-Wide Association Study Provides Insights in the Genetic Architecture and Heritability of Long QT Syndrome. Circulation. 2020;142(4):324-338.
Matsuda S, Ohnuki Y, Okami M, Ochiai E, Yamada S, Takahashi K, et al. Jervell and Lange-Nielsen syndrome with novel KCNQ1 and additional gene mutations. Hum Genome Var. 2020;7(1):34.
Sharma N, Cortez D, Disori K, Imundo JR, Beck M. A Review of Long QT Syndrome: Everything a Hospitalist Should Know. Hosp Pediatr. 2020;10(4):369-375.
Modell SM, Bradley DJ, Lehmann MH. Genetic testing for long QT syndrome and the category of cardiac ion channelopathies. PLoS Curr [Internet]. 2012 [citado 16 de febrero de 2021];4. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3392134/
Faridi R, Tona R, Brofferio A, Hoa M, Olszewski R, Schrauwen I, et al. Mutational and phenotypic spectra of KCNE1 deficiency in Jervell and Lange‐Nielsen Syndrome and Romano‐Ward Syndrome. Hum Mutat. 2019;40(2):162-176.
Oertli A, Rinné S, Moss R, Kääb S, Seemann G, Beckmann B-M, et al. Molecular Mechanism of Autosomal Recessive Long QT-Syndrome 1 without Deafness. Int J Mol Sci. 2021;22(3):1112.
Ono M, Burgess DE, Schroder EA, Elayi CS, Anderson CL, January CT, et al. Long QT Syndrome Type 2: Emerging Strategies for Correcting Class 2 KCNH2 (hERG) Mutations and Identifying New Patients. Biomolecules 2020;10(8):1144.
Pérez AR, Barbosa R, Daminello R, Da Costa de Rezende M, Esposito IC, De Abreu LC. The congenital long QT syndrome Type 3: An update. Indian Pacing Electrophysiol J. 2018;18(1):25-35.
Wallace E, Howard L, Liu M, O’Brien T, Ward D, Shen S, et al. Long QT Syndrome: Genetics and Future Perspective. Pediatr Cardiol. 2019;40(7):1419-1430.
Medeiros A, Iturralde P, Ackerman MJ. Clinical and Genetic Characteristics of Long QT Syndrome. Rev Esp Cardiol Engl Ed. 2007;60(7):739-752.
Roberts JD, Asaki SY, Mazzanti A, Bos JM, Tuleta I, Muir AR, et al. An International Multicenter Evaluation of Type 5 Long QT Syndrome: A Low Penetrant Primary Arrhythmic Condition. Circulation 2020;141(6):429-439.
Veerapandiyan A, Statland JM, Tawil R. Andersen-Tawil Syndrome. En: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Mirzaa G, et al., editores. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993 [citado 16 de febrero de 2021]. Disponible en: http://www.ncbi.nlm.nih.gov/books/NBK1264/
Kukla P, Biernacka E, Baranchuk A, Jastrzebski M, Jagodzinska M. Electrocardiogram in Andersen-Tawil Syndrome. New Electrocardiographic Criteria for Diagnosis of Type-1 Andersen-Tawil Syndrome. Curr Cardiol Rev. 2014;10(3):222-228.
Mellor GJ, Panwar P, Lee AK, Steinberg C, Hathaway JA, Bartels K, et al. Type 8 long QT syndrome: pathogenic variants in CACNA1C-encoded Cav1.2 cluster in STAC protein binding site. EP Eur. 2019;21(11):1725-1732.
Sepp R, Hategan L, Bácsi A, Cseklye J, Környei L, Borbás J, et al. Timothy syndrome 1 genotype without syndactyly and major extracardiac manifestations. Am J Med Genet A. 2017;173(3):784-789.
Cutler MJ, Kaufman ES. To Be or Not to Be: Long-QT Syndrome Type 9. Circ Cardiovasc Genet. 2013;6(5):439-440.
Wang F, Liu J, Hong L, Liang B, Graff C, Yang Y, et al. The phenotype characteristics of type 13 long QT syndrome with mutation in KCNJ5 (Kir3.4-G387R). Heart Rhythm 2013;10(10):1500-1506.
Waddell-Smith KE, Skinner JR. Update on the Diagnosis and Management of Familial Long QT Syndrome. Heart Lung Circ. 2016;25(8):769-776.
Garson A, Dick M, Fournier A, Gillette PC, Hamilton R, Kugler JD, et al. The long QT syndrome in children. An international study of 287 patients. Circulation 1993;87(6):1866-1872.
Bazett HC. An analysis of the time-relations of electrocardiograms. Ann Noninvasive Electrocardiol. 1997;2(2):177-194.
Cobos Gil MA, García Rubira JC. Who Was the Creator of Bazett’s Formula? Rev Esp Cardiol Engl Ed. 2008;61(8):896-897.
Fridericia LS. Die Systolendauer im Elektrokardiogramm bei normalen Menschen und bei Herzkranken. Acta Med Scand. 2009;53(1):469-486.
Hodges M. Rate Correction of the QT Interval. CEPR 1997;1(3):360-363.
Extramiana F, Maison-Blanche P, Badilini F. Circadian Modulation of QT Rate Dependence in Healthy Volunteers Gender and Age Differences. Journal of Electrocardiology. 1999;32(1):33-43.
Mauriello DA, Johnson JN, Ackerman MJ. Holter Monitoring in the Evaluation of Congenital Long QT Syndrome: Holter monitoring in LQTS. Pacing Clin Electrophysiol. 2011;34(9):1100-1104.
Vilcant V, Kousa O, Hai O. Implantable Loop Recorder. En: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 [citado 16 de febrero de 2021]. Disponible en: http://www.ncbi.nlm.nih.gov/books/NBK470398/
Schwartz PJ, Crotti L. QTc Behavior During Exercise and Genetic Testing for the Long-QT Syndrome. Circulation 2011;124(20):2181-2184.
Churet M, Luttoo K, Hocini M, Haïssaguerre M, Sacher F, Duchateau J. Diagnostic reproducibility of epinephrine drug challenge interpretation in suspected long QT syndrome. J Cardiovasc Electrophysiol. 2019;30(6):896-901.
Goldenberg I, Moss AJ. Long QT Syndrome. J Am Coll Cardiol. 2008;51(24):2291-2300.
Márquez MF. El síndrome de QT largo: una breve revisión del diagnóstico electrocardiográfico incluyendo la prueba de Viskin. Arch Cardiol México 2012;82(3):243-247.
Viskin S, Postema PG, Bhuiyan ZA, Rosso R, Kalman JM, Vohra JK, et al. The Response of the QT Interval to the Brief Tachycardia Provoked by Standing. J Am Coll Cardiol. 2010;55(18):1955-1961.
Krahn AD, Yee R, Chauhan V, Skanes AC, Wang J, Hegele RA, et al. Beta blockers normalize QT hysteresis in long QT syndrome. Am Heart J. 2002;143(3):528-534.
Muñoz C, Zorio E, Domingo D, Peñafiel P, Sánchez- JJ, García E, et al. Valor del «test de bipedestación» en el diagnóstico y la evaluación de la respuesta al tratamiento con bloqueadores beta en el síndrome de QT largo. Rev Esp Cardiol. 2017;70(11):907-914.
Gu B, Liu T, Yang L, Zhang H, Xin Y, Wang J. Head-up tilt test induces T-wave alternans in long QT syndrome with KCNQ1 gene mutation: Case report CARE-compliant article. Medicine (Baltimore) 2020;99(20):e19818.
Marrakchi S, Kammoun I, Bennour E, Laroussi L, Ben Miled M, Kachboura S. Inherited primary arrhythmia disorders: cardiac channelopathies and sports activity. Herz 2020;45(2):142-157.
Abu-Zeitone A, Peterson DR, Polonsky B, McNitt S, Moss AJ. Efficacy of Different Beta-Blockers in the Treatment of Long QT Syndrome. J Am Coll Cardiol. 2014;64(13):1352-1358.
Chockalingam P, Crotti L, Girardengo G, Johnson JN, Harris KM, Van der Heijden JF, et al. Not All Beta-Blockers Are Equal in the Management of Long QT Syndrome Types 1 and 2. J Am Coll Cardiol. 2012;60(20):2092-2099.
Han L, Liu F, Li Q, Qing T, Zhai Z, Xia Z, et al. The Efficacy of Beta-Blockers in Patients With Long QT Syndrome 1–3 According to Individuals’ Gender, Age, and QTc Intervals: A Network Meta-analysis. Front Pharmacol. 2020;11:579525.
Waddell-Smith KE, Li J, Smith W, Crawford J, Skinner JR. β-Blocker Adherence in Familial Long QT Syndrome. Circ Arrhythm Electrophysiol. 2016;9(8):e003591.
Chorin E, Hu D, Antzelevitch C, Hochstadt A, Belardinelli L, Zeltser D, et al. Ranolazine for Congenital Long-QT Syndrome Type III: Experimental and Long-Term Clinical Data. Circ Arrhythm Electrophysiol. 2016;9(10):004370.
Cano J, Zorio E, Mazzanti A, Arnau MÁ, Trenor B, Priori SG, et al. Ranolazine as an Alternative Therapy to Flecainide for SCN5A V411M Long QT Syndrome Type 3 Patients. Front Pharmacol. 2020;11:580481.
Chorin E, Taub R, Medina A, Flint N, Viskin S, Benhorin J. Long-term flecainide therapy in type 3 long QT syndrome. EP Eur. 2018;20(2):370-376.
Sakata S, Kurata Y, Li P, Notsu T, Morikawa K, Miake J, et al. Instability of KCNE1-D85N that Causes Long QT Syndrome: Stabilization by Verapamil. Pacing Clin Electrophysiol. 2014;37(7):853-863.
Pick JM, Batra AS. Implantable cardioverter-defibrillator implantation for primary and secondary prevention: indications and outcomes. Cardiol Young 2017;27(S1):126-131.
DeWitt ES, Abrams DJ. Implantable cardioverter-defibrillators in children. Arch Dis Child. 2015;100(3):265-270.
Gonzalez MC, Sieira J, Pappaert G, De Asmundis C, Chierchia GB, La Meir M, et al. Implantable Cardioverter-Defibrillators in Children and Adolescents With Brugada Syndrome. J Am Coll Cardiol. 2018;71(2):148-157.
Asakai H, Shimizu A, Mitsuhashi T, Ueyama T, Yokoshiki H, Nishii N, et al. Current Trends in Implantable Cardioverter-Defibrillator Therapy in Children ― Results From the JCDTR Database ―. Circ J. 2018;83(1):52-55.
Molina MJ, Álvarez M, Tercedor L. Desfibrilador implantable, ¿la solución definitiva? Cardiocore 2016;51(3):104-107.
Rhodes T, Weiss R. Device Therapy in the Setting of Long QT Syndrome. Card Electrophysiol Clin. 2015;7(3):479-486.
Kriebel T, Müller MJ, Ruschewski W, Krause U, Paul T, Schneider H. Value of Regular Defibrillation Threshold Testing After Extracardiac Implantable Cardioverter Defibrillator Placement in Small Children During Mid-Term Follow-Up. JACC Clin Electrophysiol. 2018;4(7):936-943.
Haag MB, Hersh AR, Toffey DE, Sargent JA, Stecker EC, Heitner SB, et al. Cost-Effectiveness of Implantable Cardioverter-Defibrillators in Children with Cardiac Conditions Associated with Risk for Sudden Cardiac Death. Pediatr Cardiol. 2020;41(7):1484-1491.
Cardiac sympathetic denervation: evolving technique, expanding indications [Internet]. [Citado 17 de febrero de 2021]. Disponible en: https://esc365.escardio.org/Congress/176009-cardiac-sympathetic-denervation-evolving-technique-expanding-indications
Lampridis S, Antonopoulos A, Kakos C, Mitsos S, Patrini D, Lawrence DR, et al. Video-thoracoscopic left cardiac sympathetic denervation for long-QT syndrome. Asian Cardiovasc Thorac Ann. 2020;0(0):1-5.
Li K, Yang J, Guo W, Lv T, Guo J, Li J, et al. Video-Assisted Thoracoscopic Left Cardiac Sympathetic Denervation in Chinese Patients with Long QT Syndrome. Int Heart J. 2018;59(6):1346-1351.
Anand S, Jain V, Agarwala S, Sachdeva S, Kothari S. Thoracoscopic left cardiac sympathetic denervation in a child with refractory long QT syndrome. J Indian Assoc Pediatr Surg. 2019;24(4):297.
Anderson H, Bos J, Rohatgi R, Ackerman M. The Effect of Left Cardiac Sympathetic Denervation on Exercise in Patients With Long QT Syndrome. JACC Clin Electrophysiol. 2019;5(9):1084-1090.
Dusi V, De Ferrari GM, Pugliese L, Schwartz PJ. Cardiac Sympathetic Denervation in Channelopathies. Front Cardiovasc Med. 2019;6(27)1-13.
Dusi V, Ajijola OA. Cardiopulmonary Performance After Left Cardiac Sympathetic Denervation for Long QT Syndromes. JACC Clin Electrophysiol. 2019;5(9):1091-1092.
Han J, Ackerman MJ, Moir C, Cai C, Xiao P-L, Zhang P, et al. Left cardiac sympathetic denervation reduces skin sympathetic nerve activity in patients with long QT syndrome. Heart Rhythm 2020;17(10):1639-1645.
Mingyang X, Weijie C, Yuehui Y. Renal denervation for treating congenital long QT syndrome: shortening the QT interval or modulating sympathetic tone?—Author’s reply. EP Eur. 2019;21(11):1756-1757.
Akkuş M, Seyrek Y, Kafalı HC, Ergül Y. Bilateral cardiac sympathetic denervation in children with long-QT syndrome and catecholaminergic polymorphic ventricular tachycardia. J Electrocardiol. 2020;61:32-36.