Las arritmias en la amiloidosis cardiaca
DOI:
https://doi.org/10.47487/apcyccv.v3i2.217Palabras clave:
Amiloidosis, Arritmias Cardíacas, ElectrofisiologíaResumen
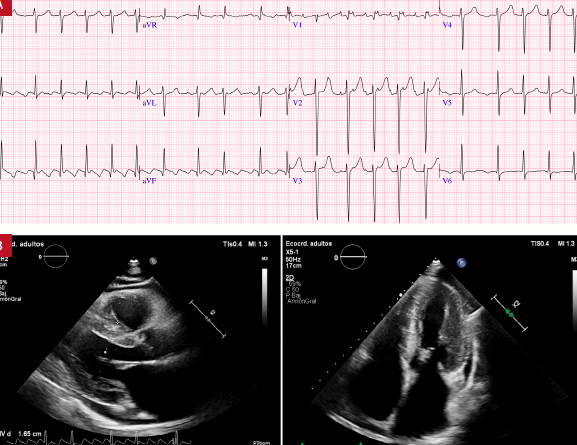
La amiloidosis cardíaca (AC) es una forma de cardiomiopatía caracterizada por el depósito extracelular de fibrillas de proteínas en el miocardio, lo que produce insuficiencia cardíaca, arritmias y alteraciones en el sistema de conducción eléctrica. La mayoría de las cardiomiopatías tienen una estrecha relación con las alteraciones del ritmo cardiaco, en especial la AC que está asociada a diferentes formas de arritmias, incluso en fases previas al diagnóstico. Arritmias como la fibrilación auricular se observan hasta en el 70% de los pacientes con AC asociadas a un especial riesgo de complicaciones cardioembólicas independiente de la estratificación de riesgo. Las arritmias ventriculares son frecuentes; sin embargo, la colocación del cardiodesfibrilador implantable no ha demostrado mejorar la sobrevida. La enfermedad del sistema de conducción eléctrica también es común, y con frecuencia es necesario implantar un marcapaso definitivo, incluso en pacientes asintomáticos. En esta revisión delimitamos las recomendaciones de las guías más recientes, resumimos datos históricos y contemporáneos, y describimos estrategias basadas en evidencia para el manejo de arritmias y sus secuelas en la AC.
Descargas
Referencias
Ovidio P. Metamorfosis. Edición Álvarez y Rosa Consuelo. Vigésimo segunda edición. Madrid, España: Ediciones Cátedra; 2021.
Gertz MA, Dispenzieri A, Sher T. Pathophysiology and treatment of cardiac amyloidosis. Nat Rev Cardiol. 2015;12(2):91-102. doi: 10.1038/nrcardio.2014.165.
Giancaterino S, Urey MA, Darden D, Hsu J. Management of Arrhythmias in Cardiac Amyloidosis. JACC Clin Electrophysiol. 2020;6(4):351-361. doi: 10.1016/j.jacep.2020.01.004.
Quock TP, Yan T, Chang E, Guthrie S, Broder M. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. doi: 10.1182/bloodadvances.2018016402.
Lane T, Fontana M, Martinez-Naharro A, Quarta C, WhelanC, Petrie A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16-26. doi: 10.1161/CIRCULATIONAHA.118.038169.
Sanchis K, Cariou E, Colombat M, Ribes D, Huart A, Cintas P, et al. Atrial fibrillation and subtype of atrial fibrillation in cardiac amyloidosis: clinical and echocardiographic features, impact on mortality. Amyloid. 2019;26(3):128-138. doi: 10.1080/13506129.2019.1620724.
Barbhaiya CR, Kumar S, Baldinger SH, Michaud G, Stevenson W, Falk R, et al. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm. 2016;13(2):383-90. doi: 10.1016/j.hrthm.2015.09.016.
Goldsmith YB, Liu J, Chou J, Hoffman J, Comenzo R, Steingart R. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol. 2009;104(7):990-4. doi: 10.1016/j.amjcard.2009.05.040.
Boldrini M, Salinaro F, Mussinelli R, Raimondi A, Alogna A, Musca F, et al. Prevalence and prognostic value of conduction disturbances at the time of diagnosis of cardiac AL amyloidosis. Ann Noninvasive Electrocardiol. 2013;18(4):327-35. doi: 10.1111/anec.12032.
Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, Falk RH. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91(2):141-57. doi: 10.1093/qjmed/91.2.141.
Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114(7):1089-93. doi: 10.1016/j.amjcard.2014.07.026.
Kristen AV, Perz JB, Schonland SO, Hegenbart U, Schnabel PA, Kristen JH, et al. Non-invasive predictors of survival in cardiac amyloidosis. Eur J Heart Fail. 2007;9(6-7):617-24. doi: 10.1016/j.ejheart.2007.01.012.
Henein MY, Suhr OB, Arvidsson S, Pilebro B, Westermarck P, Hörnstein R, et al. Reduced left atrial myocardial deformation irrespective of cavity size: a potential cause for atrial arrhythmia in hereditary transthyretin amyloidosis. Amyloid. 2018;25(1):46-53. doi: 10.1080/13506129.2018.1430027.
Leone O, Boriani G, Chiappini B, Pacini D, Cenacchi G, Suarez M, et al. Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation. Eur Heart J. 2004;25(14):1237-41. doi: 10.1016/j.ehj.2004.04.007.
Longhi S, Quarta CC, Milandri A, Lorenzini M, Gaglierdi C, Manuzzi L, et al. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid. 2015;22(3):147-55. doi: 10.3109/13506129.2015.1028616.
Dale Z, Chandrashekar P, Al-Rashdan L, Gill S, Elman M, Fischer K, et al. Routine ambulatory heart rhythm monitoring for detection of atrial arrhythmias in transthyretin cardiac amyloidosis. Int J Cardiol. 2022;358:65-71. doi: 10.1016/j.ijcard.2022.04.045.
Papathanasiou M, Jakstaite AM, Oubari S, Siebermair J, Wakili R, Hoffmann J, et al. Clinical features and predictors of atrial fibrillation in patients with light-chain or transthyretin cardiac amyloidosis. ESC Heart Fail. 2022;9(3):1740-1748. doi: 10.1002/ehf2.13851.
Fernandes F, Alencar Neto AC, Bueno BVK, Cafezeiro CRF, Rissato JH, Szor RS, et al. Clinical, Laboratory, and Imaging Profile in Patients with Systemic Amyloidosis in a Brazilian Cardiology Referral Center. Arq Bras Cardiol. 2022;118(2):422-432. doi: 10.36660/abc.20201003.
Martinez-Naharro A, Gonzalez-Lopez E, Corovic A, Mirelis J, Baksi A, Moon J, et al. High Prevalence of Intracardiac Thrombi in Cardiac Amyloidosis. J Am Coll Cardiol. 2019;73(13):1733-1734. doi: 10.1016/j.jacc.2019.01.035.
Donnellan E, Elshazly MB, Vakamudi S, Wazni O, Cohen J, Kanj M, et al. No association between CHADS-VASc score and left atrial appendage thrombus in patients with transthyretin amyloidosis. JACC Clin Electrophysiol. 2019;5(12):1473-1474. doi: 10.1016/j.jacep.2019.10.013.
Roberts WC, Waller BF. Cardiac amyloidosis causing cardiac dysfunction: analysis of 54 necropsy patients. Am J Cardiol. 1983;52(1):137-46. doi: 10.1016/0002-9149(83)90084-x.
Palmer C, Truong VT, Slivnick JA, Wolking S, Coleman P, Mazur W, et al. Atrial function and geometry differences in transthyretin versus immunoglobulin light chain amyloidosis: a cardiac magnetic resonance study. Sci Rep. 2022;12(1):140. doi: 10.1038/s41598-021-03359-9.
El-Am EA, Dispenzieri A, Melduni RM, Ammash N, White R, Hodge D, et al. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol. 2019;73(5):589-597. doi: 10.1016/j.jacc.2018.10.079.
Cariou E, Sanchis K, Rguez K, Blanchard V, Cazalbou S, Fournier P, et al. New Oral Anticoagulants vs. Vitamin K Antagonists Among Patients with Cardiac Amyloidosis: Prognostic Impact. Front Cardiovasc Med. 2021;8:742428. doi: 10.3389/fcvm.2021.742428. eCollection 2021.
Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63(6):1285-8. doi: 10.1161/01.cir.63.6.1285.
Gertz MA, Skinner M, Connors LH, Falk H, CohenA, Kyle R. Selective binding of nifedipine to amyloid fibrils. Am J Cardiol. 1985;55(13 Pt 1):1646. doi: 10.1016/0002-9149(85)90996-8.
Mints YY, Doros G, Berk JL, Connors L, Ruberg F. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail. 2018;5(5):772-779. doi: 10.1002/ehf2.12308.
Donnellan E, Wazni O, Kanj M, Eishazly M, Hussein A, Baranowski B, et al. Atrial fibrillation ablation in patients with transthyretin cardiac amyloidosis. Europace. 2020;22(2):259-264. doi: 10.1093/europace/euz314.
Rochlani YM, Nishi SN, Hakeem A, Bhatti S. Burden of arrhythmias in patients hospitalized with cardiac amyloidosis. Circulation. 2015;132:A20097. doi: 10.1161/circ.132.suppl_3.20097.
Kristen AV, Dengler TJ, Hegenbart U, Schonland S,Golschmidt H, Sack F, et al. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008;5(2):235-40. doi: 10.1016/j.hrthm.2007.10.016.
Ridolfi RL, Bulkley BH, Hutchins GM. The conduction system in cardiac amyloidosis: clinical and pathologic features of 23 patients. Am J Med. 1977;62(5):677-86. doi: 10.1016/0002-9343(77)90870-1.
Li R, Yang Z-g, Wen L-y, Liu X, XU H, Zhang Q, et al. Regional myocardial microvascular dysfunction in cardiac amyloid light-chain amyloidosis: assessment with 3T cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2016;18:16. doi: 10.1186/s12968-016-0240-7.
Sayed RH, Rogers D, Khan F, Wechalekar A, Lachmann H, Fontana M, et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J. 2015;36(18):1098-105. doi: 10.1093/eurheartj/ehu506.
Pagourelias ED, Mirea O, Duchenne J, Van Cleemput J, Delforge M, Bogaert J, et al. Echo parameters for differential diagnosis in cardiac amyloidosis: a head-to-head comparison of deformation and nondeformation parameters. Circ Cardiovasc Imaging. 2017;10(3):e005588. doi: 10.1161/CIRCIMAGING.116.005588.
Dawson DK, Hawlisch K, Prescott G, Roussin I, Di Pietro E, Deac M, et al. Prognostic role of CMR in patients presenting with ventricular arrhythmias. JACC Cardiovasc Imaging. 2013;6(3):335-44. doi: 10.1016/j.jcmg.2012.09.012.
Alkindi S, Almasoud A, Younes A, Elamm C, Benatti R, Oliveira G, et al. Increased risk of heart block in patients with cardiac amyloidosis on amiodarone. J Card Fail. 2015;21(8):S125. doi: 10.1016/j.cardfail.2015.06.359.
Maurer MS, Schwartz JH, Gundapaneni B, Elliot P, Merlini G, Waddington M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007-116. doi: 10.1056/NEJMoa1805689.
Varr BC, Zarafshar S, Coakley T, Liedtke M, Lafayette R, Arai S, et al. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. 2014;11(1):158-62. doi: 10.1016/j.hrthm.2013.10.026.
Hamon D, Algalarrondo V, Gandjbakhch E, Extramiana F, Marijon E, Elbaz N, et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. 2016;222:562-568. doi: 10.1016/j.ijcard.2016.07.254.
John RM, Stern DL. Use of implantable electronic devices in patients with cardiac amyloidosis. Can J Cardiol. 2020;36(3):408-415. doi: 10.1016/j.cjca.2019.12.002.
Alreshq R, Tugal D, Siddiqi O, Ruberg F. Conduction abnormalities and role of cardiac pacing in cardiac amyloidosis: A systematic review. Pacing Clin Electrophysiol. 2021;44(12):2092-2099. doi: 10.1111/pace.14375.
Donnellan E, Wazni OM, Saliba WI, Hanna M, Kanj M, Patel D, et al. Prevalence, incidence, and impact on mortality of conduction system disease in transthyretin cardiac amyloidosis. Am J Cardiol. 2020;128:140-146. doi: 10.1016/j.amjcard.2020.05.021.
Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203-12. doi: 10.1161/CIRCULATIONAHA.108.843334.
González-López E, Gallego-Delgado M, Guzzo-Merello G, Haro-Del Moral F, Cobo-Marcos M, Robles C, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585-94. doi: 10.1093/eurheartj/ehv338.
Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16(11):e301-e372. doi: 10.1016/j.hrthm.2019.05.007.
Algalarrondo V, Dinanian S, Juin C, Chemla D, Bennani SL, Sebag C, et al. Prophylactic pacemaker implantation in familial amyloid polyneuropathy. Heart Rhythm. 2012;9(7):1069-75. doi: 10.1016/j.hrthm.2012.02.033.
Rehorn MR, Loungani RS, Black-Maier E, Coniglio A, Karra R, Pokorney S, et al. Cardiac implantable electronic devices: a window into the evolution of conduction disease in cardiac amyloidosis. JACC Clin Electrophysiol. 2020;6(9):1144-1154. doi: 10.1016/j.jacep.2020.04.020.
Hartnett J, Jaber W, Maurer M, Sperry B, Hanna M, Collier P, et al. Electrophysiological Manifestations of Cardiac Amyloidosis. JACC CardioOncol. 2021;3(4):506-515. doi: 10.1016/j.jaccao.2021.07.010.
Chamarthi B, Dubrey SW, Cha K, Skinner M, Falk RH. Features and prognosis of exertional syncope in light-chain associated AL cardiac amyloidosis. Am J Cardiol. 199;80(9):1242-5. doi: 10.1016/s0002-9149(97)00653-x.
Delahaye N, Le Guludec D, Dinanian S, Delforge J, Slama MS, Sarda L, et al. Myocardial muscarinic receptor upregulation and normal response to isoproterenol in denervated hearts by familial amyloid polyneuropathy. Circulation. 2001;104(24):2911-6. doi: 10.1161/hc4901.100380.
Publicado
Número
Sección
Licencia
Derechos de autor 2022 La revista es titular de la primera publicación, luego el autor dando crédito a la primera publicación.

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.




















