Valor pronóstico del descenso absoluto de la porción N-terminal del propéptido natriurético tipo B en insuficiencia cardiaca descompensada: análisis secundario del estudio CLUSTER-HF
DOI:
https://doi.org/10.47487/apcyccv.v3i1.198Palabras clave:
Insuficiencia cardiaca, Péptido natriurético encefálico, HospitalizaciónResumen
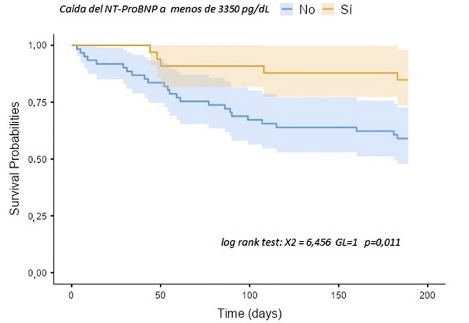
Objetivo. Determinar el valor pronóstico del descenso absoluto de la porción N-terminal del propéptido natriurético tipo B (NT–proBNP) para prevenir menos eventos clínicos en la población del estudio CLUSTER-HF (eficacia del ultrasonido pulmonar para guiar la terapia y prevenir las rehospitalizaciones en insuficiencia cardiaca). Materiales y métodos. El presente estudio fue realizado en un subgrupo de 94 pacientes con información disponible de NT-proBNP al alta hospitalaria y previo a la aleatorización del estudio CLUSTER-HF. El objetivo primario del estudio fue determinar el valor pronóstico de descenso absoluto de NT - pro-BNP por debajo del cual se prediga menos eventos de muerte por todas las causas, visitas a urgencias y rehospitalización por insuficiencia cardiaca a 180 días. Resultados. El descenso absoluto de NT-proBNP por debajo de 3 350 pg/mL tiene una capacidad discriminativa moderada con un AUC= 0,602, con un valor pronóstico significativo en el evento combinado a 180 días (log rank test, p=0,01). Asimismo, de acuerdo al análisis multivariado es un marcador independiente de eventos clínicos a 180 días OR 0,319 (0,102-0,995, p=0,04) por encima de otras variables clínicas. Conclusiones. El tener un descenso absoluto menor a 3 350 pg/mL de NT - pro-BNP al alta de la hospitalización por insuficiencia cardiaca se asoció a menos eventos clínicos a 180 días.
Descargas
Referencias
Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021 Mar 1:S1071-9164(21)00050-6. doi: 10.1016/j.cardfail.2021.01.022.
Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11(12):e004873. doi: 10.1161/CIRCHEARTFAILURE.117.004873.
Tromp J, Bamadhaj S, Cleland JGF, Angermann CE, Dahlstrom U, Ouwerkerk W, et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Health. 2020;8(3):e411-22. doi: 10.1016/S2214-109X(20)30004-8.
Girerd N, Seronde M-F, Coiro S, Chouihed T, Bilbault P, Braun F, et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. 2018;6(4):273-85. doi: 10.1016/j.jchf.2017.09.023.
Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61(4):391-403. doi: 10.1016/j.jacc.2012.09.038.
Cleland JGF, Hindricks G, Petrie M. The shocking lack of evidence for implantable cardioverter defibrillators for heart failure; with or without cardiac resynchronization. Eur Heart J. 2019;40(26):2128-30. doi: 10.1093/eurheartj/ehz409.
Bettencourt P, Januzzi JL Jr. Amino-terminal pro-B-type natriuretic peptide testing for inpatient monitoring and treatment guidance of acute destabilized heart failure. Am J Cardiol. 2008;101(3A):67-71. doi: 10.1016/j.amjcard.2007.11.026.
Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168-74. doi: 10.1161/01.CIR.0000144310.04433.BE.
Stienen S, Salah K, Eurlings LWM, Bettencourt P, Pimenta JM, Metra M, et al. Challenging the two concepts in determining the appropriate pre-discharge N-terminal pro-brain natriuretic peptide treatment target in acute decompensated heart failure patients: absolute or relative discharge levels? Eur J Heart Fail. 2015;17(9):936-44. doi: 10.1002/ejhf.320.
Metra M, Nodari S, Parrinello G, Specchia C, Brentana L et al. The role of plasma biomarkers in acute heart failure. Serial changes and independent prognostic value of NT-proBNP and cardiac troponin-T. Eur J Heart Fail. 2007;9(8):776-86. doi: 10.1016/j.ejheart.2007.05.007.
Araiza-Garaygordobil D, Gopar-Nieto R, Martinez-Amezcua P, Cabello-López A, Alanis-Estrada G, Luna-Herbert A, et al. A randomized controlled trial of lung ultrasound-guided therapy in heart failure (CLUSTER-HF study). Am Heart J. 2020;227:31-9. doi: 10.1016/j.ahj.2020.06.003.
Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JGF, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824-39. doi: 10.1016/j.ejheart.2008.07.014.
Wettersten N. Biomarkers in acute heart failure: Diagnosis, prognosis, and treatment. Int J Heart Fail. 2021;3(2):81. doi: 10.36628/ijhf.2020.0036.
Bettencourt P, Ferreira S, Azevedo A, Ferreira A. Preliminary data on the potential usefulness of B-type natriuretic peptide levels in predicting outcome after hospital discharge in patients with heart failure. Am J Med. 2002;113(3):215-9. doi: 10.1016/s0002-9343(02)01184-1.
Cheng V, Kazanagra R, Garcia A, Lenert L, Krishnaswamy P, Gardetto N, et al. A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386-91. doi: 10.1016/s0735-1097(00)01157-8.
Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of readmission after decompensated heart failure. J Am Coll Cardiol [Internet]. 2004 Feb 18;43(4):635-41. doi: 10.1016/j.jacc.2003.09.044.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239. doi: 10.1016/j.jacc.2013.05.019.
Salah K, Kok WE, Eurlings LW, Bettencourt P, Pimenta JM, Metra M, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart. 2014;100(2):115-25. doi: 10.1136/heartjnl-2013-303632.
Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019 Jun;21(6):715-31. doi: 10.1002/ejhf.1494.
Miglioranza MH, Gargani L, Sant’Anna RT, Rover MM, Martins VM, Mantovani A, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6(11):1141-51. doi: 10.1016/j.jcmg.2013.08.004.
Bitar Z, Maadarani O, Almerri K. Sonographic chest B-lines anticipate elevated B-type natriuretic peptide level, irrespective of ejection fraction. Ann Intensive Care. 2015;5(1):56. doi: 10.1186/s13613-015-0100-x.
Stienen S, Salah K, Moons AH, Bakx AL, van Pol P, Kortz RAM, et al. NT-proBNP (N-Terminal pro-B-Type Natriuretic Peptide)-Guided Therapy in Acute Decompensated Heart Failure: PRIMA II Randomized Controlled Trial (Can NT-ProBNP-Guided Therapy During Hospital Admission for Acute Decompensated Heart Failure Reduce Mortality and Readmissions?). Circulation. 2018;137(16):1671-83. doi: 10.1161/CIRCULATIONAHA.117.029882.
Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2017;318(8):713-20. doi: 10.1001/jama.2017.10565.