Gap between availability and utilization of cardiac donors: analysis of determining factors in Ecuador
DOI:
https://doi.org/10.47487/apcyccv.v6i4.530Keywords:
Transplantation, Tissue and Organ Procurement, Heart Transplantation, Health Care RationingAbstract
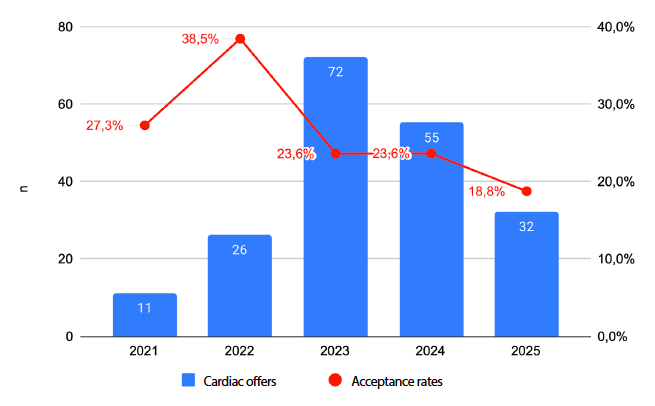
Objectives. To analyse factors associated with the rejection of heart donor offers referred to Clínica Guayaquil and to compare these findings with national data from the Instituto Nacional de Donación y Trasplante de Órganos, Tejidos y Células, in order to identify trends influencing organ acceptance. Materials and Methods. We conducted an observational, retrospective study of heart donor offers received between September 2021 and July 2025. Demographic, clinical, anthropometric, and logistical variables were extracted from the institutional database and the National Information System for Donation and Transplantation (SINIDOT). Reasons for organ rejection were classified into eight predefined categories. Univariate and multivariate analyses were performed to identify factors associated with donor acceptance or rejection. Results. A total of 196 heart donor offers were received, of which 75% were rejected. Accepted donor organs were from younger donors, were more frequently male, and had higher predicted heart mass (PHM). Traumatic brain injury was the leading cause of death (49.5%). The most common reasons for rejection were classification as a non-standard risk donor (39.5%), logistical constraints (30.6%), and blood group incompatibility (15.6%). In multivariate analyses, older donor age and origin outside Guayaquil were associated with higher rejection rates, whereas male sex and higher PHM were associated with increased acceptance. Logistical problems rose from 0% in 2021 to more than 40% in 2024-2025, largely driven by limited availability of air transport. Conclusions. The high rate of donor heart rejection reflects substantial underutilisation of potentially viable organs. Strengthening transport logistics and broadening donor acceptance criteria could increase graft utilisation, reduce waiting-list mortality, and improve the overall efficiency of national heart transplantation programmes.
Downloads
References
El Rafei A, Cogswell R, Atik FA, Zuckermann A, Allen LA. Review of the Global Activity of Heart Transplant. Circ Heart Fail. 2025;18(7):e012272. doi: 10.1161/CIRCHEARTFAILURE.124.012272.
Kupiec-Weglinski JW. Grand Challenges in Organ Transplantation. Front Transplant. 2022;1:897679. doi: 10.3389/frtra.2022.897679.
Instituto Nacional de Donación y Trasplante de Órganos, Tejidos y Células. Informe de rendición de cuentas 2022 [Internet]. Quito; 2023 [citado el 7 de agosto de 2025]. Disponible en: http://www. donaciontrasplante.gob.ec/indot/rendicion-de-cuentas-2022/
Global Observatory on Donation and Transplantation. Summary - Ecuador. GODT; 2022 [citado el 7 de agosto de 2025]. Disponible en: https://www.transplant-observatory.org/ summary/
Instituto Nacional de Donación y Trasplante de Órganos, Tejidos y Células. Informe de rendición de cuentas 2021 [Internet]. Quito; 2022 [citado el 7 de agosto de 2025]. Disponible en: http://www. donaciontrasplante.gob.ec/indot/rendicion-de-cuentas-2021/
Instituto Nacional de Donación y Trasplante de Órganos, Tejidos y Células. Informe de rendición de cuentas 2024 [Internet]. Quito; 2025 [citado el 7 de agosto de 2025]. Disponible en: https://www.donaciontrasplante.gob.ec/indot/rendicion-decuentas-2024/
Jain R, Kransdorf EP, Cowger J, Jeevanandam V, Kobashigawa JA. Donor Selection for Heart Transplantation in 2025. JACC Heart Fail. 2025;13(3):389-401. doi: 10.1016/j.jchf.2024.09.016.
Copeland H, Knezevic I, Baran DA, Rao V, Pham M, Gustafsson F, et al. Donor heart selection: Evidence-based guidelines for providers. J Heart Lung Transplant. 2023;42(1):7-29. doi: 10.1016/j.healun.2022.08.030
Masarone D, Kittleson MM, Falco L, Martucci ML, Catapano D, Brescia B, et al. The ABC of Heart Transplantation—Part 1: Indication, Eligibility, Donor Selection, and Surgical Technique. J Clin Med. 2023;12(16):5217. doi: 10.3390/jcm12165217.
Cheung A, Toma M. Clinical Guidelines for Adult Heart Transplantation in British Columbia [Internet]. British Columbia; 2022 [citado el 7 de agosto de 2025]. Disponible en: https://www.transplant.bc.ca/Documents/Health%20 Professionals/Clinical%20guidelines/Heart-Transplant-ClinicalGuidelines-2022.pdf
Chamorro-Jambrina C, Silva-Obregón JA, MartínezMelgar JL, Romera-Ortega MÁ. Donor Selection for Heart Transplantation. JACC Heart Fail. 2025;13(8):102501. doi: 10.1016/j.jchf.2025.03.043.
Instituto Nacional de Donación y Trasplante de Órganos, Tejidos y Células. Informe de rendición de cuentas 2023 [Internet]. Quito; 2024 [citado el 7 de agosto de 2025]. Disponible en: http://www. donaciontrasplante.gob.ec/indot/rendicion-de-cuentas-2023/
Mahillo Durán B. Calidad y seguridad del donante de riesgo no estándar [Internet]. Madrid: Universidad Complutense de Madrid; 2021 [citado el 14 de agosto de 2025]. Disponible en: http://hdl.handle.net/20.500.14352/5460
Instituto Nacional de Donación y Trasplante de Órganos, Tejidos y Células. Formulario INDOT-PDC-02 [Internet]. Quito; 2014 [citado el 19 de noviembre de 2025]. Disponible en: http:// www.donaciontrasplante.gob.ec/indot/wp-content/uploads/ downloads/2014/01/FORMULARIO_INDOT-PDC-02.pdf
Asemota N, Louca J, Oechsner M, Williams L, Messer S, Manara A, et al. A critical evaluation of donor heart offer acceptance in the United Kingdom. Ann Cardiothorac Surg. 2025;14(1):37-46. doi: 10.21037/acs-2024-dcd-24.
Khush KK, Luikart H, Neidlinger N, Salehi A, Nguyen J, Geraghty PJ, et al. Challenges encountered in conducting donor-based research: Lessons learned from the Donor Heart Study. Am J Transplant. 2022;22(7):1760-5. doi: 10.1111/ajt.17051.
Wayda B, Weng Y, Zhang S, Luikart H, Pearson T, Nieto J, et al. Prediction of Donor Heart Acceptance for Transplant and Its Clinical Implications: Results from The Donor Heart Study. Circ Heart Fail. 2024;17(10):e011360. doi: 10.1161/ CIRCHEARTFAILURE.123.011360.
Parra Puerto JA, Rangel Rivera DA. Características clínicas y diferencias de tamaño donante-receptor en trasplante cardíaco de adultos. Bogotá: Universidad del Rosario; 2023.
Israni AK, Zaun D, Gauntt K, Schaffhausen C, McKinney W, Snyder JJ. OPTN/SRTR 2020 Annual Data Report: DOD. Am J Transplant. 2022;22(S2):519-52. doi: 10.1111/ajt.16976.
Dharmavaram N, Hess T, Jaeger H, Smith J, Hermsen J, Murray D, et al. National Trends in Heart Donor Usage Rates: Are We Efficiently Transplanting More Hearts? J Am Heart Assoc. 2021;10(15):e019655. doi: 10.1161/JAHA.120.019655.
T. Jenkins R, M. Shah M, L. Larson E, L. Zhou A, M. Ruck J, Kilic A. Expanding the Criteria for Heart Transplantation Donors: A Review of DCD, Increased Ischemic Times, HCV, HIV, and Extended Criteria Donors. Heart Surg Forum. 2023;26(5):E639-55. doi: 10.59958/hsf.6677.
Baran DA, Long A, Lansinger J, Copeland JG, Copeland H. Donor Utilization in the Recent Era: Effect of Sex, Drugs, and Increased Risk. Circ Heart Fail. 2022;15(7):e009547. doi: 10.1161/ CIRCHEARTFAILURE.122.009547.
Wang Y, Cai J, Sun Y, Zhang J, Xie F, Alshirbini MH, et al. Extended donor criteria in heart transplantation: a retrospective study from a single Chinese institution. J Thorac Dis. 2018;10(4):2153- 65. doi: 10.21037/jtd.2018.03.149.
Bakhtiyar SS, Sakowitz S, Verma A, Chervu NL, Benharash P. Expanded Criteria Donor Heart Allograft Utilization: National Trends and Outcomes. Ann Thorac Surg. 2023;116(6):1250-8. doi: 10.1016/j.athoracsur.2023.09.013.
Gobierno de la República del Ecuador, Ministerio de Salud Pública del Ecuador. Tarifario de prestaciones para el sistema nacional de salud. Quito: Ministerio de Salud Pública; 2014.
Latam-Airlines Ecuiador S.A.; Instituto Nacional de Donación y Transplante de Órganos, Tejidos y Células. Convenio de Donación de Boletos Aéreos entre Latam-Airlines Ecuador S.A. – Instituto Nacional de Donación y Transplante de Órganos, Tejidos y Células [Internet]. Ecuador: República del Ecuador; 2021 [citado el 19 de noviembre de 2025]. http://www. donaciontrasplante.gob.ec/indot/lotaip/2024/mayo/varios/ convenio_indot_latam-signed.pdf
Avianca Ecuador S.A.; Instituto Nacional de Donación y Transplante de Órganos, Tejidos y Células. Convenio de Colaboración Suscrito entre Avianca Ecuador S.A. y el Instituto Nacional de Donación y Transplante de Órganos, Tejidos y Células “INDOT” [Internet]. Ecuador: República del Ecuador; 2022 [citado el 19 de noviembre de 2025]. http://www. donaciontrasplante.gob.ec/indot/lotaip/2024/mayo/varios/ Convenio%20traslado%20organos%20y%20personal%20 medico%20Indot%20-%20Avianca%20Ecuador%20(vf).pdf
Organ Transport Working Group. Organ Transport Working Group final report [Internet]. Washington, DC; 2025 [citado el 8 de agosto de 2025]. Disponible en: https://www.faa.gov/about/ office_org/headquarters_offices/avs/offices/afx/afs/afs200/ organ_transport/Organ_Transport_Working_Group_Final_ Report.pdf
D’Alessandro DA, Zuckermann A. Moderate controlled hypothermia vs. standard ice-cold storage of cardiac allografts to expand the donor pool: insights from the GUARDIAN registry. Ann Cardiothorac Surg. 2025;14(1):28-36. doi: 10.21037/acs2024-dcd-21.
Stehlik J, Farr MA, Mehra MR, Schroder JN, D’Alessandro DA, Pal JD, et al. Clinical Outcomes with Normothermic Pulsatile Organ Perfusion in Heart Transplantation: A Report from the OCS Heart Perfusion Registry. Circulation. 2025;151(13):896-909. doi: 10.1161/CIRCULATIONAHA.124.071743.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 The journal is headline of the first publication, then the author giving credit to the first publication.

This work is licensed under a Creative Commons Attribution 4.0 International License.














