Vaccines in cardiology, an underutilized strategy to reduce the residual cardiovascular risk
DOI:
https://doi.org/10.47487/apcyccv.v5i1.349Keywords:
Vaccines, Influenza, Pneumonia, Pneumococcal, Herpes Zoster, COVID-19, Respiratory Syncytial Virus, Human, PreventionAbstract
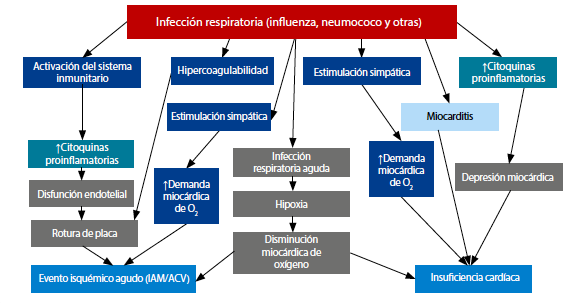
Cardiovascular diseases stand as the leading cause of mortality among adults globally. For decades, comprehensive evidence has underscored the correlation between infections, particularly those involving the respiratory system, and an elevated risk of cardiovascular and cerebrovascular events, as well as all-cause mortality. The mechanisms through which infections heighten cardiovascular events are intricate, encompassing immune system activation, systemic inflammation, hypercoagulable states, sympathetic system activation, and increased myocardial oxygen demand. Respiratory infections further contribute hypoxemia to this complex interplay. These mechanisms intertwine, giving rise to endothelial dysfunction, plaque ruptures, myocardial depression, and heart failure. They can either instigate de novo cardiovascular events or exacerbate pre-existing conditions. Compelling evidence supports the safety of influenza, pneumococcal, herpes zoster, COVID-19 and respiratory syncytial virus vaccines in individuals with cardiovascular risk factors or established cardiovascular disease. Notably, the influenza vaccine has demonstrated safety even when administered during the acute phase of a myocardial infarction in individuals undergoing angioplasty. Beyond safety, these vaccinations significantly reduce the incidence of cardiovascular events in individuals with an augmented cardiovascular risk. Nevertheless, vaccination rates remain markedly suboptimal. This manuscript delves into the intricate relationship between infections and cardiovascular events. Additionally, we highlight the role of vaccinations as a tool to mitigate these occurrences and reduce residual cardiovascular risk. Finally, we emphasize the imperative need to optimize vaccination rates among individuals with heart diseases.
Downloads
References
Garcia-Zamora S, Sosa Liprandi MI, Picco JM, Matta MG, Villarreal R, Pulido L, et al. [Immunizations in adults with cardiovascular disease. Summary of the Consensus of the Argentine Cardiology Society]. Medicina (B Aires). 2020;80(5):541-53. Spanish.
Roth GA, Mensah GA, Fuster V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J Am Coll Cardiol. 2020;76(25):2980-1. doi: 10.1016/j.jacc.2020.11.021.
Dugani S, Gaziano TA. 25 by 25: Achieving Global Reduction in Cardiovascular Mortality. Curr Cardiol Rep. 2016;18(1):10. doi: 10.1007/s11886-015-0679-4.
Everett BM. Residual Inflammatory Risk: A Common and Important Risk Factor for Recurrent Cardiovascular Events. J Am Coll Cardiol. 2019;73(19):2410-2. doi: 10.1016/j.jacc.2019.02.056.
Mattila KJ. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med. 1989;225(5):293-6. doi: 10.1111/ j.1365-2796.1989.tb00084.x.
Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611-8. doi: 10.1056/ NEJMoa041747.
Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125(6):773-81. doi: 10.1161/ CIRCULATIONAHA.111.040766.
Sethi NJ, Safi S, Korang SK, Hrobjartsson A, Skoog M, Gluud C, et al. Antibiotics for secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2021;2(2):CD003610. doi: 10.1002/14651858.CD003610.pub4.
Warren-Gash C, Bhaskaran K, Hayward A, Leung GM, Lo SV, Wong CM, et al. Circulating influenza virus, climatic factors, and acute myocardial infarction: a time series study in England and Wales and Hong Kong. J Infect Dis. 2011;203(12):1710-8. doi: 10.1093/infdis/ jir171.
Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51(3):1701794. doi: 10.1183/13993003.01794-2017.
Vardeny O, Claggett B, Udell JA, Packer M, Zile M, Rouleau J, et al. Influenza Vaccination in Patients with Chronic Heart Failure: The PARADIGM-HF Trial. JACC Heart Fail. 2016;4(2):152-8. doi: 10.1016/j. jchf.2015.10.012.
Matta MG, Pulido L, Herrera-Paz JJ, Picco JM, Wolff S, Tse G, et al. Influenza and pneumococcal vaccine prescription for adults during COVID-19 first wave in three regions of Argentina. Vaccine. 2023;41(9):1541-4. doi: 10.1016/j.vaccine.2023.01.056.
Sosa Liprandi A, Zaidel EJ, López Santi R, Araujo JJ, Banos González A, Busso JM, et al. Influenza and Pneumococcal Vaccination in Non-Infected Cardiometabolic Patients from the Americas during the COVID-19 Pandemic. A Sub-Analysis of the CorCOVID-LATAM Study. Vaccines (Basel). 2021;9(2):123. doi: 10.3390/vaccines9020123.
Gurfinkel EP, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. Eur Heart J. 2004;25(1):25- 31. doi: 10.1016/j.ehj.2003.10.018.
Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310(16):1711-20. doi: 10.1001/jama.2013.279206.
Yedlapati SH, Khan SU, Talluri S, Lone AN, Khan MZ, Khan MS, et al. Effects of Influenza Vaccine on Mortality and Cardiovascular Outcomes in Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2021;10(6):e019636. doi: 10.1161/JAHA.120.019636.
Frobert O, Gotberg M, Erlinge D, Akhtar Z, Christiansen EH, MacIntyre CR, et al. Influenza Vaccination After Myocardial Infarction: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Circulation. 2021;144(18):1476-84. doi: 10.1161/ CIRCULATIONAHA.121.057042.
GrohskopfLA,BlantonLH,FerdinandsJM,ChungJR,BroderKR,Talbot HK, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2022-23 Influenza Season. MMWR Recomm Rep. 2022;71(1):1-28. doi: 10.15585/mmwr.rr7101a1.
Luna CM, Pulido L, Rizzo O, Gauna ML, Chirino A, Videla AJ. [Recomendaciones actualizadas para la vacunación de adultos con enfermedades respiratorias. Asociación Argentina de Medicina Respiratoria, 2023]. Medicina (B Aires). 2023 [in press].
Tamerius J, Nelson MI, Zhou SZ, Viboud C, Miller MA, Alonso WJ. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect. 2011;119(4):439-45. doi: 10.1289/ehp.1002383.
Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153.
Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9.
Gravenstein S, McConeghy KW, Saade E, Davidson HE, Canaday DH, Han L, et al. Adjuvanted Influenza Vaccine and Influenza Outbreaks in US Nursing Homes: Results from a Pragmatic Cluster-Randomized Clinical Trial. Clin Infect Dis. 2021;73(11):e4229-e36. doi: 10.1093/cid/ciaa1916.
Johansen ND, Modin D, Nealon J, Samson S, Salamand C, Larsen CS, et al. Feasibility of randomizing Danish citizens aged 65-79 years to high-dose quadrivalent influenza vaccine vs. standard-dose quadrivalent influenza vaccine in a pragmatic registry-based setting: rationale and design of the DANFLU-1 Trial. Pilot Feasibility Stud. 2022;8(1):87. doi: 10.1186/s40814-022-01044-w.
SteinAN,MillsC,McGovernI,DeanA,BogdanovA,SullivanS,HaagM. Superior Effectiveness of Cell-Based Versus Egg-Based Quadrivalent Influenza Vaccines Against Outpatient Test-Confirmed Influenza Over Three Consecutive Seasons in the United States (V130_67RWE); Proceedings of the 9th ESWI Influenza Conference; Valencia, Spain. 17–20 September 2023.
Vardeny O, Kim K, Udell JA, Joseph J, Desai AS, Farkouh ME, et al. Effect of High-Dose Trivalent vs Standard-Dose Quadrivalent Influenza Vaccine on Mortality or Cardiopulmonary Hospitalization in Patients With High-risk Cardiovascular Disease: A Randomized Clinical Trial. JAMA. 2021;325(1):39-49. doi: 10.1001/jama.2020.23649.
McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868-78. doi: 10.1016/j. jaci.2015.07.048.
Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O’Brien KL, Team AAPBS, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8(4):e60273. doi: 10.1371/journal. pone.0060273.
Essink B, Sabharwal C, Cannon K, Frenck R, Lal H, Xu X, et al. Pivotal Phase 3 Randomized Clinical Trial of the Safety, Tolerability, and Immunogenicity of 20-Valent Pneumococcal Conjugate Vaccine in Adults Aged >/=18 Years. Clin Infect Dis. 2022;75(3):390-8. doi: 10.1093/cid/ciab990.
Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, et al. Intervals Between PCV13 and PPSV23 Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-7. doi: 10.15585/mmwr.mm6434a4.
Jiménez Ruiz CA, Buljubasich D, Sansores R, Riesco Miranda JA, Guerreros Benavides A, Luhning S, et al. SEPAR-ALAT Consensus Document on Antipneumoccal Vaccination in Smokers. Arch Bronconeumol. 2015;51(7):350-4. doi: 10.1016/j.arbres.2014.12.003.
Marques Antunes M, Duarte GS, Brito D, Borges M, Costa J, Ferreira JJ, et al. Pneumococcal vaccination in adults at very high risk or with established cardiovascular disease: systematic review and meta- analysis. Eur Heart J Qual Care Clin Outcomes. 2021;7(1):97-106. doi: 10.1093/ehjqcco/qcaa030.
ArvinA.Aging,immunity,andthevaricella-zostervirus.NEnglJMed. 2005;352(22):2266-7. doi: 10.1056/NEJMp058091.
Curran D, Callegaro A, Fahrbach K, Neupane B, Vroling H, van Oorschot D, et al. Meta-Regression of Herpes Zoster Incidence Worldwide. Infect Dis Ther. 2022;11(1):389-403. doi: 10.1007/s40121- 021-00567-8.
Sundstrom K, Weibull CE, Soderberg-Lofdal K, Bergstrom T, Sparen P, Arnheim-Dahlstrom L. Incidence of herpes zoster and associated events including stroke--a population-based cohort study. BMC Infect Dis. 2015;15:488. doi: 10.1186/s12879-015-1170-y.
AddarioA,CelarierT,BongueB,BarthN,GavazziG,Botelho-NeversE. Impact of influenza, herpes zoster, and pneumococcal vaccinations on the incidence of cardiovascular events in subjects aged over 65
years: a systematic review. Geroscience. 2023;45(6):3419-47. doi: 10.1007/s11357-023-00807-4.
Warren-Gash C. Herpes Zoster: Epidemiological Links With Stroke and Myocardial Infarction. J Infect Dis. 2018;218(suppl_2):S102-S6.
Erskine N, Tran H, Levin L, Ulbricht C, Fingeroth J, Kiefe C, et al. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS One. 2017;12(7):e0181565. doi: 10.1371/journal.pone.0181565.
Seo HM, Cha MJ, Han JH, Han K, Park SH, Bang CH, et al. Reciprocal relationship between herpes zoster and cardiovascular diseases: A nationwide population-based case-control study in Korea. J Dermatol. 2018;45(11):1312-8. doi: 10.1111/1346-8138.14597.
Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087-96. doi: 10.1056/NEJMoa1501184.
CunninghamAL,LalH,KovacM,ChlibekR,HwangSJ,Diez-Domingo J, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med. 2016;375(11):1019-32. doi: 10.1056/ NEJMoa1603800.
StrezovaA,Diez-DomingoJ,AlShawafiK,TinocoJC,ShiM,PirrottaP, et al. Long-term Protection Against Herpes Zoster by the Adjuvanted Recombinant Zoster Vaccine: Interim Efficacy, Immunogenicity, and Safety Results up to 10 Years After Initial Vaccination. Open Forum Infect Dis. 2022;9(10):ofac485. doi: 10.1093/ofid/ofac485.
Yang Q, Chang A, Tong X, Merritt R. Herpes Zoster Vaccine Live and Risk of Stroke Among Medicare Beneficiaries: A Population-Based Cohort Study. Stroke. 2021;52(5):1712-21. doi: 10.1161/STROKEAHA.120.032788.
Helm MF, Khoury PA, Pakchanian H, Raiker R, Maczuga S, Foulke GT. Recombinant Zoster Vaccine Reduces 3-Year Cardiovascular Risk: Insights from a Multi-Centered Database. J Drugs Dermatol. 2023;22(12):1178-82. doi: 10.36849/JDD.7415.
Garcia-ZamoraS,LeeS,HaseebS,BazoukisG,TseG,Alvarez-GarciaJ, et al. Arrhythmias and electrocardiographic findings in Coronavirus disease 2019: A systematic review and meta-analysis. Pacing Clin Electrophysiol. 2021;44(6):1062-74. doi: 10.1111/pace.14247.
Garcia-ZamoraS,PiccoJM,LeporiAJ,GalelloMI,SaadAK,AyonM,et al. Abnormal echocardiographic findings after COVID-19 infection: a multicenter registry. Int J Cardiovasc Imaging. 2023;39(1):77-85. doi: 10.1007/s10554-022-02706-9.
JiangJ,ChanL,KauffmanJ,NarulaJ,CharneyAW,OhW,etal.Impact of Vaccination on Major Adverse Cardiovascular Events in Patients With COVID-19 Infection. J Am Coll Cardiol. 2023;81(9):928-30. doi: 10.1016/j.jacc.2022.12.006.
Ivey KS, Edwards KM, Talbot HK. Respiratory Syncytial Virus and Associations with Cardiovascular Disease in Adults. J Am Coll Cardiol. 2018;71(14):1574-83. doi: 10.1016/j.jacc.2018.02.013.
Feldman RG, Antonelli-Incalzi R, Steenackers K, Lee DG, Papi A, Ison MG, et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine Is Efficacious in Older Adults with Underlying Medical Conditions. Clin Infect Dis. 2024;78(1):202-9. doi: 10.1093/cid/ciad471.
Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am J Med. 2008;121(7 Suppl 2):S28-35. doi: 10.1016/j. amjmed.2008.05.005.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 The journal is headline of the first publication, then the author giving credit to the first publication.

This work is licensed under a Creative Commons Attribution 4.0 International License.














