Combined leadless pacing and subcutaneous defibrillation strategy in a high-risk patient: first case report from Peru
DOI:
https://doi.org/10.47487/apcyccv.v6i4.513Palabras clave:
Pacemaker, Artificial, Defibrillators, Heart Failure, InfectionResumen
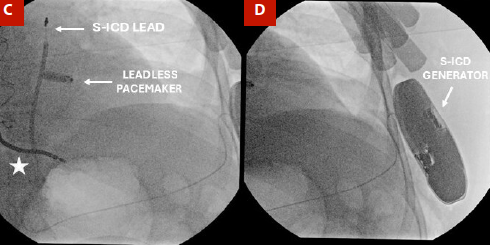
We present the case of a 51-year-old male with non-ischemic dilated cardiomyopathy and complete atrioventricular block, who was previously implanted with a cardiac resynchronization therapy defibrillator. The patient developed signs of pocket infection with a high risk of extrusion. Partial system extraction was performed, followed by 14 days of intravenous antibiotic therapy. Due to a history of ventricular fibrillation and permanent pacing dependency, and in the absence of viable transvenous access, a sequential implantation strategy was adopted using a leadless pacemaker (Micra AV, Medtronic) and a subcutaneous implantable cardioverter-defibrillator (EMBLEM, Boston Scientific). Both procedures were completed without complications, and the patient showed favorable recovery, with effective pacing, no arrhythmic recurrences, and no signs of infection at the six-month follow-up. This case illustrates the feasibility of a fully leadless approach in high-risk patients with contraindications to conventional transvenous systems.
Descargas
Referencias
Wijesuriya N, De Vere F, Mehta V, Niederer S, Rinaldi CA, Behar JM. Leadless pacing: therapy, challenges and novelties. Arrhythm Electrophysiol Rev. 2023;12:e09. doi: 10.15420/aer.2022.41.
Breeman KTN, Tjong FVY, Miller MA, Neuzil P, Dukkipati S, Knops RE, et al. Ten years of leadless cardiac pacing. J Am Coll Cardiol. 2024;84(21):2131-47. doi: 10.1016/j.jacc.2024.08.077.
Saleem-Talib S, Hoevenaars CPR, Molitor N, van Driel VJ, van der Heijden J, Breitenstein A, et al. Leadless pacing: a comprehensive review. Eur Heart J. 2025;46(21):1979-90. doi: 10.1093/eurheartj/ ehaf119.
Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43(40):3997-4126. doi: 10.1093/eurheartj/ehac262.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary. Circulation. 2018;138(13):e210-71. doi: 10.1161/CIR.0000000000000548.
Dyrbuś M, Machowicz J, Kurek A, Gąsior M, Tajstra M. The automated subcutaneous implantable cardioverter-defibrillator screening in patients with leadless pacemakers. Pacing Clin Electrophysiol. 2025;48(4):464-8. doi: 10.1111/pace.15165.
Tovia-Brodie O, Rav Acha M, Belhassen B, Gasperetti A, Schiavone M, Forleo GB, et al. Implantation of cardiac electronic devices in active COVID-19 patients: results from an international survey. Heart Rhythm. 2022;19(2):206-16. doi: 10.1016/j.hrthm.2021.10.020.
Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, et al. EHRA international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections. Europace. 2020;22(4):515-49. doi: 10.1093/ europace/euz246.
Baddour LM, Esquer Garrigos Z, Rizwan Sohail M, Havers-Borgersen E, Krahn AD, Chu VH, et al. Update on cardiovascular implantable electronic device infections and their prevention, diagnosis, and management: a scientific statement from the American Heart Association. Circulation. 2024;149(2):e201-16. doi: 10.1161/ CIR.0000000000001187.
Russo AM, Desai MY, Do MM, Butler J, Chung MK, Epstein AE, et al. ACC/AHA/ASE/HFSA/HRS/SCAI/SCCT/SCMR 2025 appropriate use criteria for implantable cardioverter-defibrillators, cardiac resynchronization therapy, and pacing. J Am Coll Cardiol. 2025;85(11):1213-85. doi: 10.1016/j.jacc.2024.11.023.
Calvagna GM, Valsecchi S. Simultaneous subcutaneous implantable cardioverter-defibrillator and leadless pacemaker implantation for patients at high risk of infection: a retrospective case series report. J Interv Card Electrophysiol. 2025;68(4):943-51. doi: 10.1007/s10840- 023-01684-9.
Budrejko S, Kempa M, Przybylski A. S-ICD implantation “tips and tricks”. Rev Cardiovasc Med. 2023;24(7):195. doi: 10.31083/j. rcm2407195.
Mitacchione G, Migliore F. Co-presence of subcutaneous implantable cardioverter-defibrillator and leadless pacemaker in highrisk infection patients: are we out of the woods? J Interv Card Electrophysiol. 2025;68(4):727-9. doi: 10.1007/s10840-023-01726-2.
Milaras N, Ntalakouras I, Archontakis S, Dourvas P, Ktenopoulos N, Klogkeri T, et al. Wireless device therapy in hypertrophic cardiomyopathy using the combination of a leadless pacemaker and a subcutaneous defibrillator: a report with 2-year follow-up of two patients. J Innov Card Rhythm Manag. 2024;15(6):5908-10. doi: 10.19102/icrm.2024.15064.
Mondésert B, Dubuc M, Khairy P, Guerra PG, Gosselin G, Thibault B. Combination of a leadless pacemaker and subcutaneous defibrillator: first in-human report. HeartRhythm Case Rep. 2015;1(6):469-71. doi: 10.1016/j.hrcr.2015.07.009.
Tjong FV, Brouwer TF, Smeding L, Kooiman KM, de Groot JR, Ligon D, et al. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace. 2016;18(11):1740-7. doi: 10.1093/europace/euv457.
Descargas
Publicado
Número
Sección
Licencia
Derechos de autor 2025 La revista es titular de la primera publicación, luego el autor dando crédito a la primera publicación.

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.

















