Irreversible cardiotoxicity induced by trastuzumab: a systematic review based on a pharmacovigilance case report
DOI:
https://doi.org/10.47487/apcyccv.v6i1.456Palabras clave:
Breast Neoplasms, Cardiotoxicity, Trastuzumab, PharmacovigilanceResumen
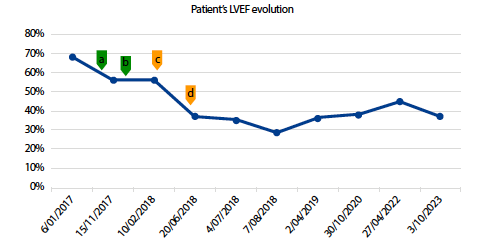
Irreversible cardiotoxicity (IC) induced by trastuzumab (TZB) is a rare but serious adverse event. As a result, its characteristics and the specific factors related to exposure remain poorly understood. This study aims to synthesize and evaluate the existing evidence on IC. We presented a pharmacovigilance case of longterm IC and conducted a systematic review (SR) of the clinical manifestations of cases reported worldwide. We reported the case using the CARE guidelines checklist and assessed the causality using the modified Algorithm of Karch and Lasagna. Following PRISMA guidelines, we conducted the SR using defined terms in PubMed, Embase, Scopus, and Web of Science from inception until June 2023. This SR included five case reports, including the pharmacovigilance case reported by us. While patients exhibited different severe clinical characteristics, receiving TZB at a 6 mg/kg dose was consistent. Despite varying treatment durations, the median time of IC diagnosis was 10 months, and the average difference between the basal and the final left ventricular ejection fraction was roughly 30%. According to the modified Karch and Lasagna algorithm, all cases were ranged from possible to probable. While TZB is generally considered a reversible cardiotoxic antineoplastic, clinicians and regulators must be aware of the potential IC risk with long-term manifestations. Vigilant cardiac monitoring and further research are crucial to better understanding and managing this serious adverse event.
Descargas
Referencias
Tiscoski KA, Giacomazzi J, Rocha MS, Gössling G, Werutsky G. Realworld data on triple-negative breast cancer in Latin America and the Caribbean. Ecancermedicalscience. 2023;17:1635. doi: 10.3332/ecancer.2023.1635.
Bouwer NI, Jager A, Liesting C, Kofflard MJM, Brugts JJ, Kitzen JJEM, et al. Cardiac monitoring in HER2-positive patients on trastuzumab treatment: A review and implications for clinical practice. Breast. 2020;52:33-44. doi: 10.1016/j.breast.2020.04.005.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229-4361. doi: 10.1093/eurheartj/ehac244.
Gabrić ID, Vazdar L, Pintarić H, Planinc D, Trbušić M, Jazvić M, et al. Prediction of reversibility of cardiotoxicity caused by immunotherapy with trastuzumab. Glob Heart. 2014 Mar;9(1 Suppl):e40. doi: 10.1016/j.gheart.2014.03.1355.
Morelli MB, Bongiovanni C, Da Pra S, Miano C, Sacchi F, Lauriola M, et al. Cardiotoxicity of anticancer drugs: Molecular mechanisms and strategies for cardioprotection. Front Cardiovasc Med. 2022;9:847012. doi: 10.3389/fcvm.2022.847012.
Mantarro S, Rossi M, Bonifazi M, D’Amico R, Blandizzi C, La Vecchia C, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med. 2016;11(1):123-40. doi: 10.1007/s11739-015-1362-x.
Yu AF, Dang CT, Jorgensen J, Moskowitz CS, DeFusco P, Oligino E, et al. Rationale and design of a cardiac safety study for reduced cardiotoxicity surveillance during HER2-targeted therapy. Cardio-Oncol. 2023;9:13. doi: 10.1186/s40959-023-00163-4.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60-3. doi: 10.1136/bmjebm-2017-110853.
Hosseini K, Fallahtafti P, Roudbari P, Soleimani H, Jahromi NA, Jameie M, et al. Spontaneous coronary artery dissection in patients with prior psychophysical stress: a systematic review of case reports and case series. BMC Cardiovasc Disord. 2024;24:235. doi: 10.1186/s12872-024-03902-2.
Tanaka S, Ikari A, Nitta T, Horiuchi T. Long-term irreversible trastuzumab-induced cardiotoxicity for metastatic breast cancer in a patient without cardiac risk factors. Oxf Med Case Rep. 2017;2017(7):omx038. doi: 10.1093/omcr/omx038.
Tham YL, Verani MS, Chang J. Reversible and Irreversible Cardiac Dysfunction Associated with Trastuzumab in Breast Cancer. Breast Cancer Res Treat. 2002;74(2):131-4. doi: 10.1023/a:1016140729725.
Skopets IS, Vezikova NN, Ivanova EP, Sergeeva SS, Ignatenko OV. A case of irreversible cardiomyopathy induced by polychemotherapy. Ter Arkh. 2015;87(12):73-6. doi: 10.17116/terarkh2015871273-76.
Bordin P, Volpe C, Adami G, Moretti V, Ermacora P. Irreversible Cardiotoxicity after Adjuvant Treatment with Trastuzumab in a Case of Breast Cancer. Tumori J. 2008;94(5):777-8. doi: 10.1177/030089160809400527.
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215-21. doi: 10.1200/JCO.2002.20.5.1215.
Calvillo-Argüelles O, Abdel-Qadir H, Suntheralingam S, Michalowska M, Amir E, Thavendiranathan P. Trastuzumab-Related Cardiotoxicity and Cardiac Care in Patients With HER2 Positive Metastatic Breast Cancer. Am J Cardiol. 2020;125(8):1270-5. doi: 10.1016/j.amjcard.2020.01.029.
Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y, et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: A meta-analysis. Cancer Treat Rev. 2011;37(4):312-20. doi: 10.1016/j.ctrv.2010.09.001.
Thavendiranathan P, Abdel-Qadir H, Fischer HD, Camacho X, Amir E, Austin PC, et al. Breast Cancer Therapy–Related Cardiac Dysfunction in Adult Women Treated in Routine Clinical Practice: A Population-Based Cohort Study. J Clin Oncol. 2016;34(19):2239-46. doi: 10.1200/JCO.2015.65.1505.
Tu CM, Chu KM, Yang SP, Cheng SM, Wang WB. Trastuzumab (Herceptin)-associated cardiomyopathy presented as new onset of complete left bundle-branch block mimicking acute coronary syndrome: a case report and literature review. Am J Emerg Med. 2009;27(7):903.e1-3. doi: 10.1016/j.ajem.2008.11.012.
Telli ML, Witteles RM. Trastuzumab-related cardiac dysfunction. J Natl Compr Canc Netw. 2011;9(2):243-9. doi: 10.6004/jnccn.2011.0019.
Bouwer NI, Steenbruggen TG, van Rosmalen J, Rier HN, Kitzen JJEM, van Bekkum ML, et al. Cardiotoxicity during long-term trastuzumab use in patients with HER2-positive metastatic breast cancer: who needs cardiac monitoring? Breast Cancer Res Treat. 2021;186(3):851-62. doi: 10.1007/s10549-020-06039-w.
Morelli M, Bongiovanni C, Da Pra S, Miano C, Sacchi F, Lauriola M, et al. Cardiotoxicity of anticancer drugs: molecular mechanisms and strategies for cardioprotection. Front Cardiovasc Med. 2022;9:847012. doi:10.3389/fcvm.2022.847012.
Zheng H, Mahmood SS, Khalique OK, Zhan H. Trastuzumab-Induced Cardiotoxicity: when and how much should we worry? JCO Oncol Pract. 2024;OP.23.00816. doi:10.1200/OP.23.00816.
Koulaouzidis G, Yung AE, Yung DE, Skonieczna-Żydecka K, Marlicz W, Koulaouzidis A, et al. Conventional cardiac risk factors associated with trastuzumab-induced cardiotoxicity in breast cancer: Systematic review and meta-analysis. Curr Probl Cancer. 2021;45(5):100723. doi:10.1016/j.currproblcancer.2021.100723.
Lee MH, Yee J, Kim YJ, Moon JY, Kim JH, Rhie SJ, et al. Factors for time to trastuzumab-induced cardiotoxicity in breast cancer patients. Med Oncol. 2017;34(12):199. doi:10.1007/s12032-017-1041-z.
Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981-8. doi:10.1161/CIRCULATIONAHA.114.013777.
Costa R, Costa-Filho RB, Talamantes SM, Queiroga Jr F, Campello EC, Cartaxo H, et al. Interstitial pneumonitis secondary to trastuzumab: a case report and literature review. Case Rep Oncol. 2017;10(2):524-30. doi:10.1159/000477340.
Descargas
Publicado
Número
Sección
Licencia
Derechos de autor 2025 La revista es titular de la primera publicación, luego el autor dando crédito a la primera publicación.

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.

















